Rumored Buzz on Dye Dilution
Wiki Article
3 Easy Facts About Dye Dilution Shown
Table of ContentsThe 25-Second Trick For Dye DilutionNot known Incorrect Statements About Dye Dilution Dye Dilution Can Be Fun For AnyoneDye Dilution for BeginnersThe Best Guide To Dye DilutionThe Only Guide to Dye DilutionThe Ultimate Guide To Dye DilutionThe Definitive Guide for Dye DilutionIndicators on Dye Dilution You Should Know
Serial dilutions are made by making the exact same dilution step over and over, using the previous dilution as the input to the next dilution in each action. Because the dilution-fold is the exact same in each step, the dilutions are a geometric series (continuous ratio between any kind of surrounding dilutions). As an example: Notification that each dilution is three-fold family member to the previous one. 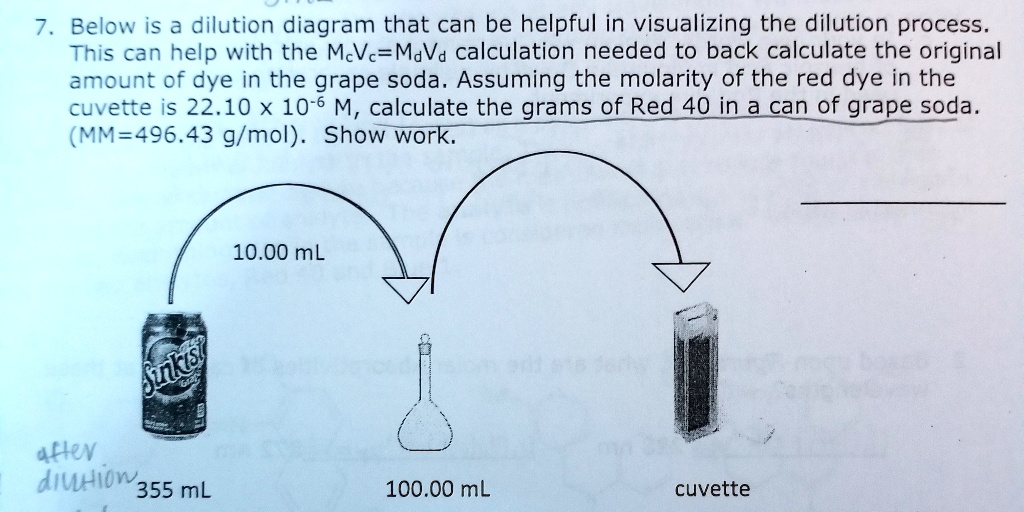
Dye Dilution for Dummies
This stays clear of bunching the majority of the factors up at one end and having just the last factor means far down the scale. Before making serial dilutions, you need to make rough estimates of the concentrations in your unknowns, and also your uncertainty in those quotes. For instance, if A280 claims you have 7.7 and also 7 mg/ml. That means you need to cover a ten-fold variety of dilutions, or maybe a bit a lot more to make sure. If the half-max of your assay happens at concerning 0. 5 mg/ml, after that your minimum dilution layer is (700 mg/ml)/(0. 5 mg/ml) = 1,400. Your optimum is (7000 mg/ml)/(0.
Rumored Buzz on Dye Dilution
So to be secure, you may want to cover 1,000 via 20,000. As a whole, prior to making a dilution collection, you require to choose: What are the cheapest as well as greatest focus (or dilutions) you require to evaluate in order to be certain of locating the half-max? These identify the series of the dilution collection.It is a lot easier to opt for 2-fold dilutions and offers about the very same result.) So, you require to make a 1/1,000 dilution to begin with. Then you need to serially thin down that 2-fold per action in five actions. You can make 1/1,000 by adding 1 microliter of sample to 0.
The Main Principles Of Dye Dilution
Why is that an inadequate selection? Because you can not gauge 1 microliter (or also 10 microliters) accurately with regular pipeters. So, make three serial 1/10 dilutions (0. 1 ml [100 microliters] right into 0. 9 ml): 1/10 x 1/10 x 1/10 = 1/1,000. Currently you can include 1. 0 ml of the beginning 1/1,000 dilution to 1.Then eliminate 1. 0 ml from that dilution (leaving 1. 0 ml for your examinations), and also include it to 1. 0 ml of diluent in the following tube (giving 1/4,000). And so forth for 3 more serial dilution actions (offering 1/8,000, 1/16,000, as well as 1/32,000). You end up with 1 (Dye Dilution). 0 ml of each discover here dilution.
Dye Dilution Fundamentals Explained
Water is the most bountiful element in the body making up about 60% of body mass in the reference guy. Because it is primarily discovered in the fat-free body in a reasonably continuous quantity, evaluation of body water has actually been of rate of interest as a method of body structure assessment for virtually 100 years.Water's particular as a singular molecular types offers itself to making use of the dilution principle, which in its easiest form, states that the volume of the part is equal to the amount of isotope contributed to the element separated by the concentration of the isotope because part. i was reading this In 1915, the dilution principle was first made use of in the research study of body make-up when the use of a red color to measure the plasma volume was theorized.
The Facts About Dye Dilution Revealed
Using a mathematical technique, a sensible quote was made to determine the quantity of plasma in which the color was initial weakened. Following this examination and also making use of the very same concept, tracer product was injected intravenously and allowed to reach a consistent circulation, and from the dilution achieved at stability, the components of the body were determined.Tritiated water was very first explained by Speed et al. as an isotope for measuring TBW - Dye Dilution. The main benefit of utilizing tritium (3H), the contaminated isotope of hydrogen, is that it is easily offered as well as easily appraised by scintillation counting. On the other hand, a huge quantity of tritiated water have to be administered to obtain sufficient precision, eliminating its usage in instances where the usage of radionuclides is limited.
The Best Strategy To Use For Dye Dilution
Greater technological mistakes have been discovered utilizing the infrared approach. When utilizing isotope dilution, specifically deuterated water, 2 body liquid examples from urine, blood, or saliva are collected: one prior to administration of the deuterium dosage to establish the natural background levels and the 2nd after permitting adequate time for penetration of the isotope.There are 4 basic assumptions that are intrinsic in any isotope dilution strategy. Tracer exchanges with nonaqueous particles are very little, and also consequently, the quantity of circulation or dilution room of the isotope can be identified, albeit a little better than the water pool.
The Best Strategy To Use For Dye Dilution

Three voids are advised after the dose when pee is used as the biological sample. The tracer is not metabolized during the equilibration time.
The Main Principles Of Dye Dilution
The inputs are stabilized by an output of water in the kind of urine, sweat, breath water, or transdermal evaporation. This continuous turn over has caused 2 techniques when evaluating TBW: the plateau technique as well as the back-extrapolation, or slope-intercept, approach. For body structure study, the plateau approach is the normal strategy.Report this wiki page